Flacon à médicament
Utiliser cette image
Puis-je réutiliser cette image sans autorisation? Oui
Les images sur le portail de la collection d’Ingenium ont la licence Creative Commons suivante :
Copyright Ingenium / CC BY-NC-ND (Attribution-NonCommercial 4.0 International (CC BY-NC 4.0)
ATTRIBUER CETTE IMAGE
Ingenium,
2021.0003.002
Permalien:
Ingenium diffuse cette image sous le cadre de licence Creative Commons et encourage son téléchargement et sa réutilisation à des fins non commerciales. Veuillez mentionner Ingenium et citer le numéro de l’artefact.
TÉLÉCHARGER L’IMAGEACHETER CETTE IMAGE
Cette image peut être utilisée gratuitement pour des fins non commerciales.
Pour un usage commercial, veuillez consulter nos frais de reproduction et communiquer avec nous pour acheter l’image.
- TYPE D’OBJET
- vaccine
- DATE
- 2020
- NUMÉRO DE L’ARTEFACT
- 2021.0003.002
- FABRICANT
- Corning Pharmaceutical Glass LLC
- MODÈLE
- Valor glass;Pfizer-BioNTech COVID 19 Vaccine
- EMPLACEMENT
- United States of America
Plus d’information
Renseignements généraux
- Nº de série
- S/O
- Nº de partie
- 2
- Nombre total de parties
- 2
- Ou
- S/O
- Brevets
- S/O
- Description générale
- Glass, metal, synthetic and vaccine residue
Dimensions
Remarque : Cette information reflète la taille générale pour l’entreposage et ne représente pas nécessairement les véritables dimensions de l’objet.
- Longueur
- S/O
- Largeur
- S/O
- Hauteur
- 3,3 cm
- Épaisseur
- S/O
- Poids
- S/O
- Diamètre
- 1,7 cm
- Volume
- S/O
Lexique
- Groupe
- Technologie médicale
- Catégorie
- Recherche
- Sous-catégorie
- S/O
Fabricant
- Ou
- Corning
- Pays
- United States of America
- État/province
- Inconnu
- Ville
- Inconnu
Contexte
- Pays
- Inconnu
- État/province
- Inconnu
- Période
- The vials, alongside orders for other countries, were carefully loaded onto a United Parcel Service airplane by staff on 12 December 2020. After stops in Germany and the United States, the shipment landed at John C. Munro Hamilton International Airport the evening of 13 December 2020.
- Canada
-
Taken from acquisition proposal "These vials were produced at a Corning facility in upstate New York. From there, they were shipped to Pfizer-BioNTech’s Belgian production facility. There, the vials were sent through an intricate and automated vaccine-filling machine where they were washed and sterilized, and filled with the Pfizer-BioNTech COVID-19 vaccine. The machine sealed them and packaged them for travel with dry ice. These vials, alongside orders for other countries, were carefully loaded onto a United Parcel Service airplane by staff on 12 December 2020. After stops in Germany and the United States, the shipment landed at John C. Munro Hamilton International Airport the evening of 13 December 2020. The University Health Network (UHN) was selected by the province to oversee the vaccine program in Toronto. Jin Huh, the senior director of pharmacy at the University Health Network, began tracking the box on 12 December at around noon. Huh received the 585 vials as part of this first delivery at 9:40 a.m. on Monday, 14 December 2021. In a carefully orchestrated workflow, he quickly moved the vials through the building, bringing them to Kelly Lalog, a registered pharmacy technician, who placed them in the – 70 C freezer. Shortly thereafter, Booth Rumsey, the pharmacy operations technician supervisor at Princess Margaret Hospital, prepared the vaccines. She inspected the vials, flipped off the deep purple dust cover cap, inverted them, and inserted a sterile syringe which diluted the vaccine with 1.8 milliliters of sodium chloride solution. After a final inversion, to keep the vaccine suspended, and inspection, she prepared five syringes, each with 0.3 ml of the vaccine. A staff member then took the five prepared syringes to the vaccine clinic at the UHN’s Michener Institute of Education. At 12:01 p.m. Tamara Dus, a registered nurse, sat down to administer the vaccines to the first five long-term-care workers to receive the jab in Canada. The UHN set aside the second vial so that they could guarantee these frontline workers would receive their booster in three weeks’ time. To mark the first vial of COVID-19 vaccine administered at UHN, Emily Musing, UHN’s chief patient safety officer, stuck a small paper circle to its bottom. She did the same for the booster vial. After the second Ontario wide stay-at-home order was lifted, Musing shipped these two vials to Ingenium." - Fonction
-
To store and transport Pfizer-BioNTech COVID-19 vaccine. - Technique
-
Taken from acquisition proposal "These vials are inextricably linked to the vaccines they contained and the significance and symbolism of their use. As such, this acquisition proposal will explore the materiality of the objects and the importance of the vaccines they once held. This section will examine the Pfizer-BioNTech COVID-19 vaccine with attention to mRNA and nanoparticle technology, Corning’s Valor Glass, and distribution. COVID-19 Vaccine: Researchers have been studying and working with mRNA vaccines for decades, although the Pfizer-BioNTech COVID-19 vaccine is the first mRNA vaccine to complete all clinical trial stages and be licensed for use. Interest has grown in these vaccines because they can be developed in a laboratory using readily available materials. mRNA vaccines have been studied before for influenza, Zika, rabies, and cytomegalovirus. Like other vaccines, those designed using messenger RNA trigger the body to produce an immune response, in this case to COVID-19. The Pfizer-BioNTech vaccine does this by introducing a mRNA sequence that produces an antigen that triggers an immune response. In other words, the vaccine provides instructions to the immune cells in the body to make a protein piece, or spike protein. When the body produces that protein, the immune system recognizes it as being foreign and produces a response against it, also known as an antibody. The benefits of mRNA vaccines are that they do not use live virus, they do not enter the nucleus of the cell, and cells break down the mRNA soon after they are finished using the instructions. (ref. 6) mRNA vaccines are generally faster and cheaper to produce than traditional vaccines, however RNA is fragile and needs to be protected; this is where nanotechnology comes into play. Lipid nanoparticles are fatty molecular envelops that help strands of mRNA, the genetic messenger for making DNA code into proteins, evade the body’s biological defence mechanisms and reach their target cell without being degraded. Research into the future applications of mRNA vaccines suggests that this technology could be a way to provide protection against multiple disease through fewer shots. It is also being explored in non-human trials for HIV antibodies, and shows promising potential as a platform for cancer immunotherapy. (ref. 7) Glass for the Pharmaceutical Industry: The glass for these vials was made by Corning, one of a handful of firms that makes glass vials for the pharmaceutical industry. Corning was founded in 1851 and, since then, it has focused its production on glass and related materials for industrial and scientific applications. Corning’s skills and knowledge in the production of quality pharmaceutical glass is an extremely important piece of this artifact. Vials that store drugs or vaccines, such as these, must meet extremely high safety standards, including being shatterproof and resistant to extraordinary temperatures. These vials are made of Valor Glass which is a relatively new pharmaceutical product from Corning. Corning began developing Valor Glass in 2011 as part of their research into improving medical vials. They experimented with different additives and found that adding new ingredients to the silica and removing standard elements made for stronger pharmaceutical glass. “Corning’s manufacturing process begins with cylindrical machines called converters. They cut and shape tubes of Valor Glass into vials, which are then submerged in a molten-salt bath. Potassium atoms in the hot mixture swap with smaller sodium atoms embedded in the surface of the glass, creating tension and therefore toughness. Corning first developed this process for Gorilla Glass, which is used in iPhones and other electronic devices; a vial fortified in this way can withstand as much as a thousand pounds of force. Afterward, the glass is rinsed, and the exterior is given a polymer coating, so that bottles don’t grind against one another on a filling line, generating glass dust that can ruin doses.” (ref. 8) Distribution: The Pfizer-BioNTech vaccine presents a few technical logistical challenges that impact how its stored and administered. The vaccine must remain at cold temperatures (between -70 C and – 80 C) up until the point of use so that the fragile RNA components keep from breaking down. This temperature requirement is a challenge for the supply chain as there is a notable lack of suitable freezer equipment, including dry ice, globally. In Canada, a number of appliances makers have stepped forward and developed news lines of freezers that can reach these hyper-cold temperatures. Danby, a compact appliance maker from Guelph, Ontario, is one such company. The Pfizer-BioNTech vaccine is highly effective at preventing COVID-19 after two doses. When the vaccines were first tested by researchers, they noticed that it produced a relatively weak immune response when given as a single dose. A much stronger immune response was identified after a second dose. With this in mind, Canada’s initial approach was to safeguard a booster dose for every individual after they received their first jab. This, understandably, greatly slowed down the vaccine rollout across the country. In the spring of 2021, however, this approach changed in response to more approved vaccines and increases in procurement. At the time of this artifact’s use, five doses were removed from each Pfizer-BioNTech vial with a three- and five-milliliter syringes for the initial dose and booster. At the request of Pfizer, Health Canada issued an independent regulatory review to determine whether each vial could supply six doses, one more than the label originally suggested. In its review, the regulator concluded that six full doses can be obtained consistently from Pfizer vials through the use of low-dead-volume syringes, which allow the user to push virtually all fluid out of the syringe chamber. This Canadian decision follows similar changes made by the European Medicines Agency and the U.S. Food and Drug Administration in 2020." - Notes sur la région
-
Inconnu
Détails
- Marques
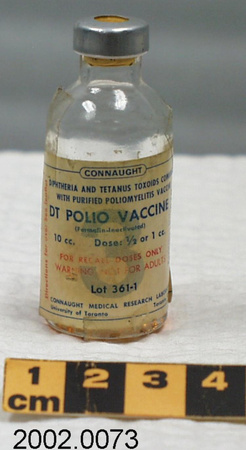
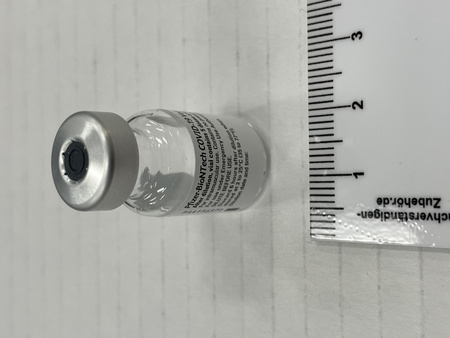
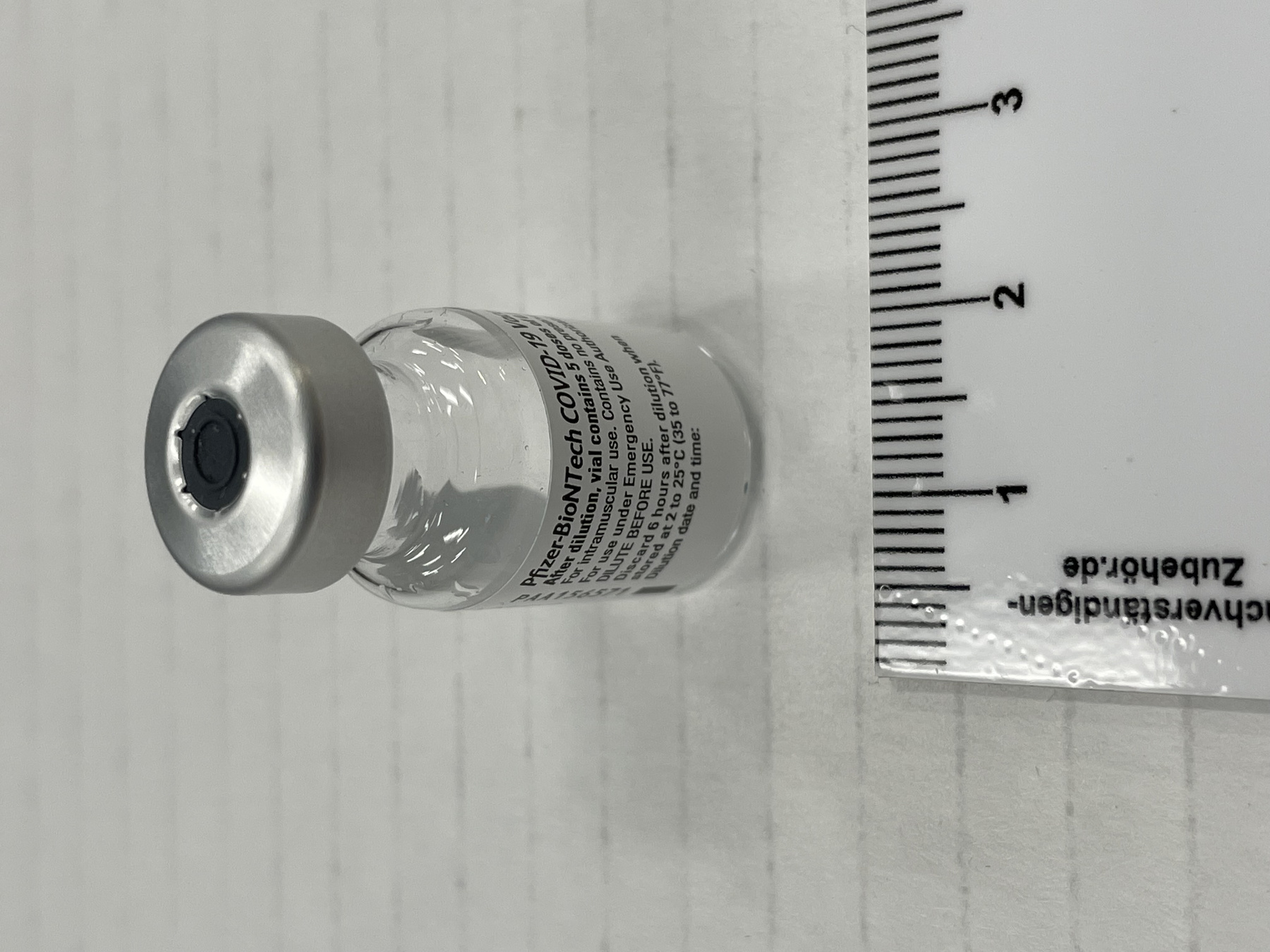
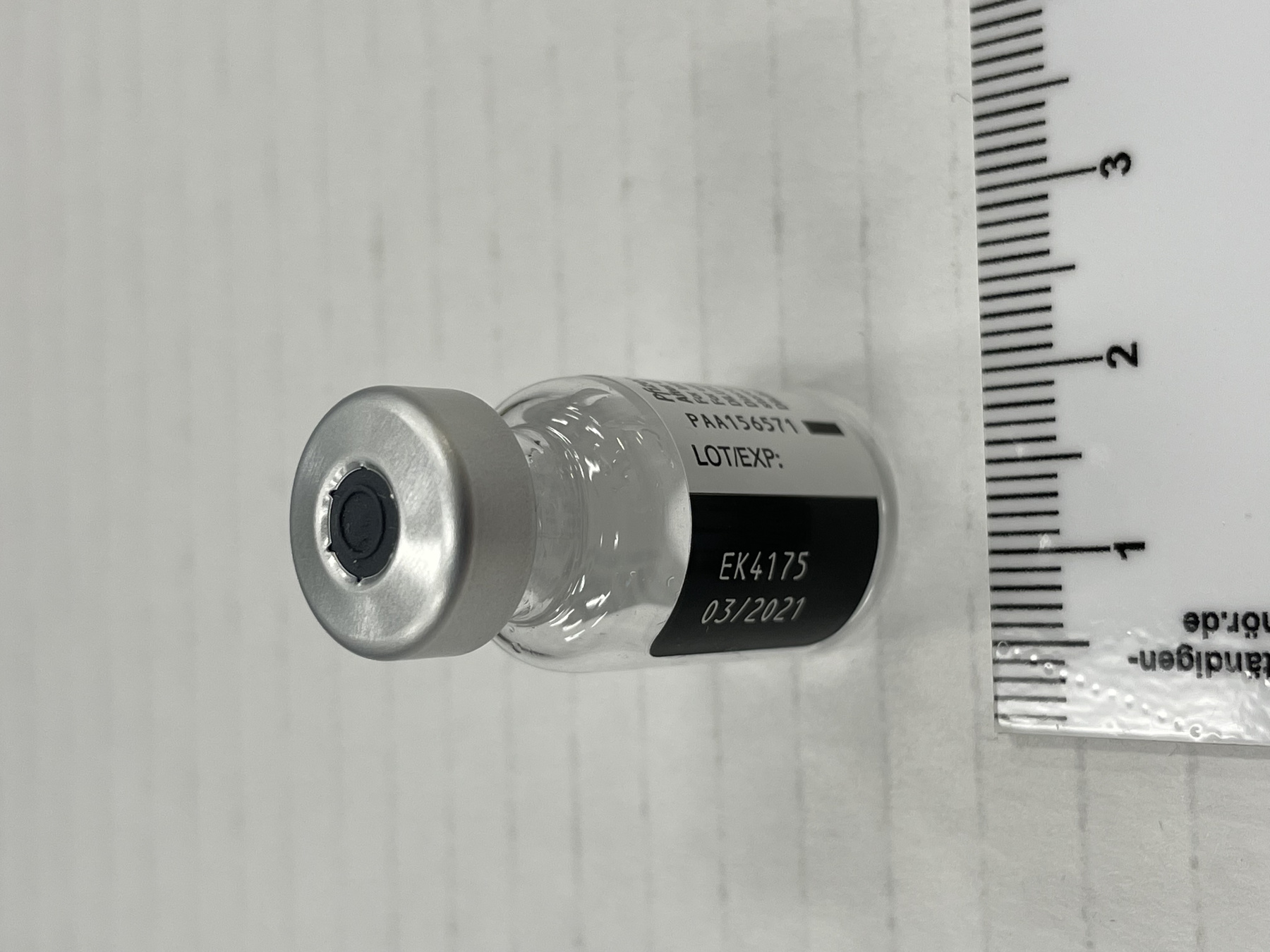
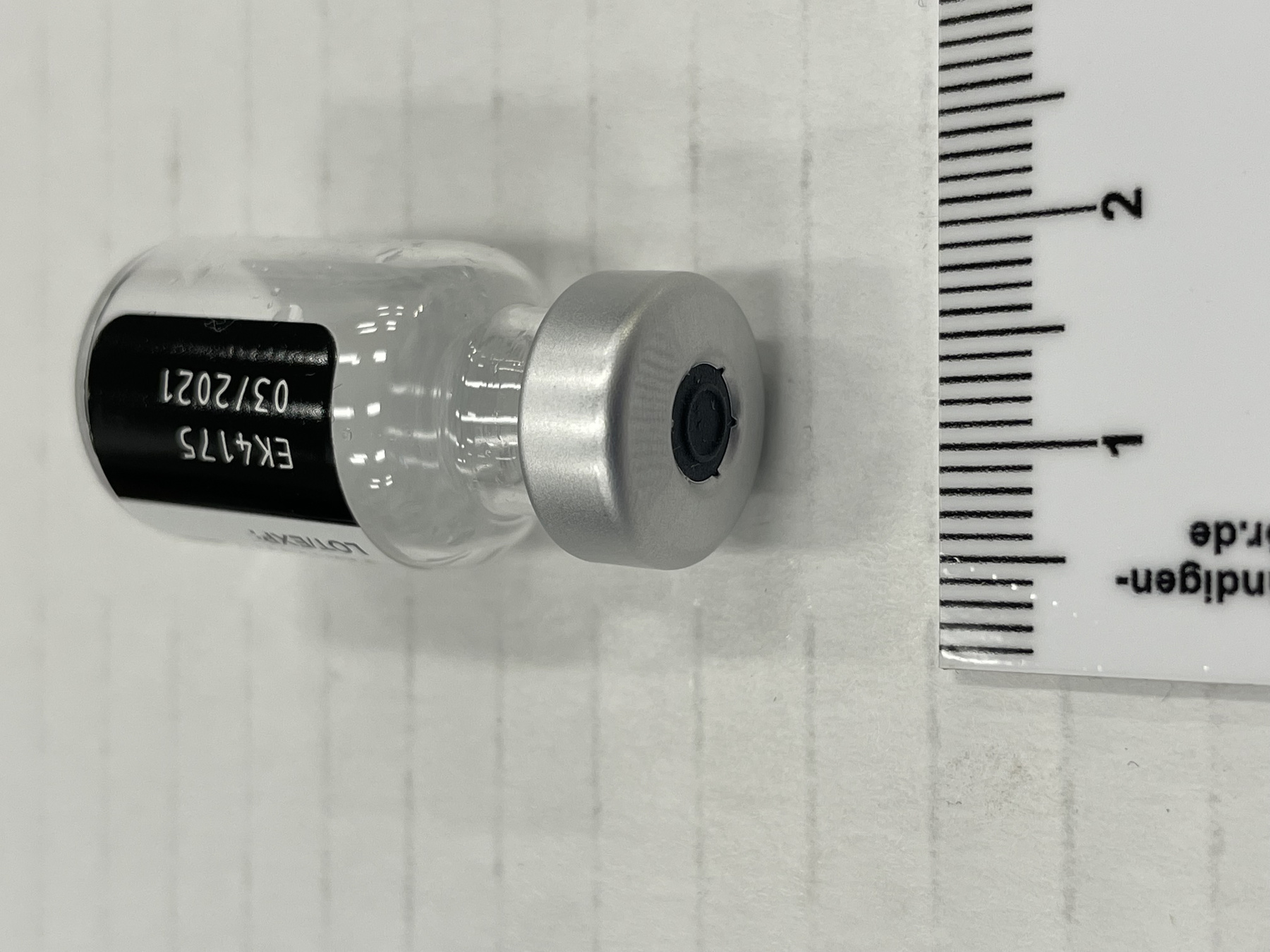
- Mfr's label reads: "Pfizer-BioNTech COVID 19 Vaccine/ After dilution, vial contains 5 doses of 0.3 ml/ For intramuscular use. Contains no preservative./ DILUTE BEFORE USE./ Discard 6 hours after dilution when/ stored at 2 to 25 (35 to 77F)./ Dilution date and time: [blank]". Written beside barcode "NDC 59267-1000-1". Written at the begining of label "PAA156571/ LOT[/]EXP: EK4175/ 03[/]2021". Green dot (sticker) on bottom of vial with a handwritten "2".
- Manque
- Appears complete
- Fini
- Clear glass bottle with a silver metal cap and a dark grey synthetic top. White label with black print. Green circular sticker on bottom of vial.
- Décoration
- S/O
FAIRE RÉFÉRENCE À CET OBJET
Si vous souhaitez publier de l’information sur cet objet de collection, veuillez indiquer ce qui suit :
Corning Pharmaceutical Glass LLC, Flacon à médicament, 2020, Numéro de l'artefact 2021.0003, Ingenium - Musées des sciences et de l'innovation du Canada, http://collection.ingenium.ca/fr/id/2021.0003.002/
RÉTROACTION
Envoyer une question ou un commentaire sur cet artefact.
Plus comme ceci